Phosphorescent paper displays a property called photoluminescence, which is a fancy way of saying "glowing." The "photo-" part of the word refers to photons, which are carriers of light. Most of us have heard of the electromagnetic spectrum, which is a way of classifying different types of radiation based on their wavelengths (the distance between two wavecrests) and their frequencies (the number of waves that pass a certain point, measured in Hertz/Hz). One fun fact is that wavelength is the inverse of frequency, which is a great thing to know if you like trivia.
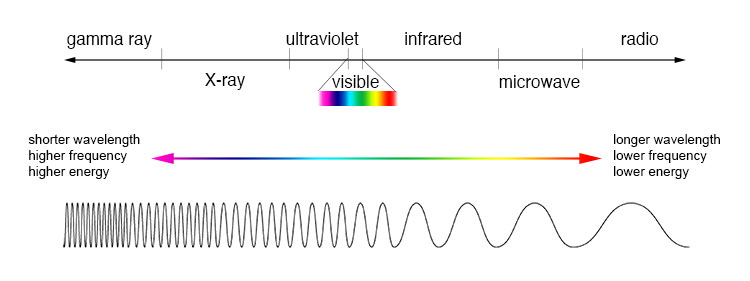 |
| The electromagnetic spectrum. From: GSFC/NASA. |
We did some qualitative (no equations, but still figuring out what's going on) experiments on the paper with some custom 3D printed Halloween cookie cutters that Don brought along with the paper. See the progression of 1) before we shined light onto it, 2) while we shined the blue flashlight onto the paper and cookie cutters, and 3) after we turned off the light and removed the cookie cutters.
 |
| Picture 1: Before we shined the blue light on the paper. PC: Jessica Noviello. |
 |
| Picture 2: While we shined the blue light on the paper. PC: Jessica Noviello. |
 |
| Picture 3: After we shined the blue light on the paper. PC: Jessica Noviello. |
What's going on here? As the photons hit the paper, the molecules that make up the paper absorb the light. All materials do this, by the way, even if it's rare to see something glow afterwards. The difference with the phosphorescent paper is, of course, the glowing after the light is removed. Different materials are more sensitive to different wavelengths of light. The paper we had was very sensitive to blue light, which is higher energy (shorter wavelength) light. It barely responded to the yellow-ish light of the nearby streetlamps and didn't notice red light (considered low energy due to its longer wavelengths).*
When the light source is removed, the molecules are slow to give up their photons. Even when the photons are released, they do not have the same energy or wavelength. This is due to something called energy states in atoms, the building blocks that make up molecules. An atom absorbing a photon is called "excited," and it shoots up an electron to a higher energy state. It's like someone perking up after having a cup of afternoon coffee. As that caffeine wears off, the energy level of the person goes back to its normal, lower, more stable state. This is what happens with the electrons when they give up their extra energy. In phosphorescent materials, it happens on a timescale we can observe.
Here are some more pictures from one of our experiments with another one of our props, a dinosaur.
 |
| PC: Jessica Noviello |
 |
| PC: Jessica Noviello |
 |
| PC: Jessica Noviello |

No comments:
Post a Comment